
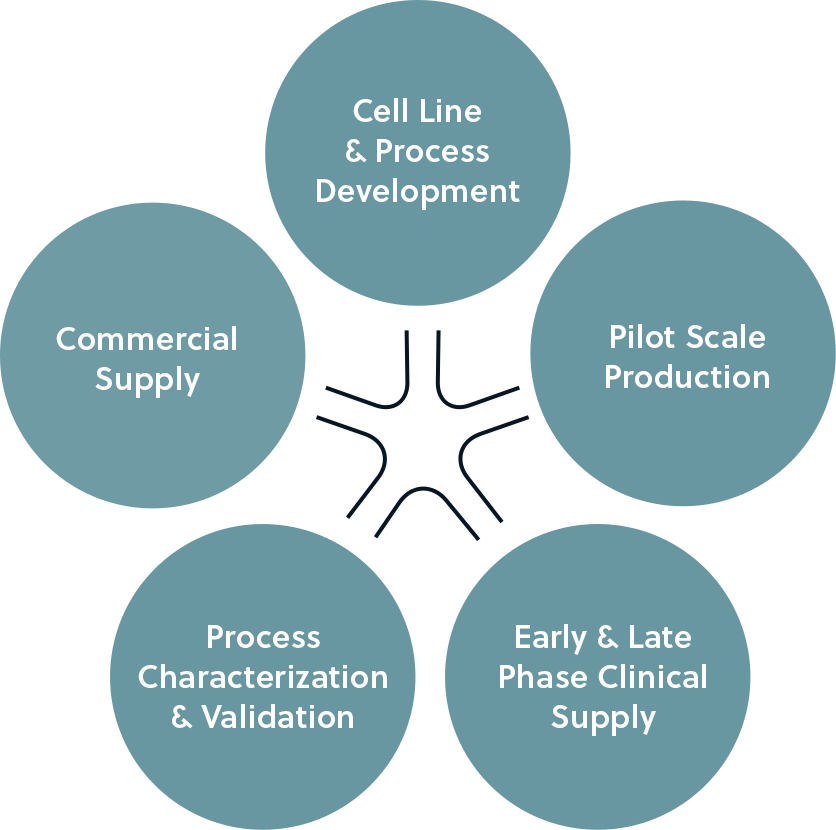
Avid Bioservices has successfully supported biosimilar development programs for nearly a decade. As a fully dedicated CDMO, we are focused on meeting the needs of our customers, and our passion for quality goes hand in hand with our excellent inspection and compliance history, providing you with peace of mind in your choice of a CDMO partner.
One of the challenges of biosimilar development is having to meet a specific set of target properties without possessing the specific information about the process used to manufacture the original drug. But even before the development process begins, you have to find a partner who can provide manufacturing services from the bench through larger-scale commercialization, with deep protein science, analytics, and process engineering expertise.
Developing Biosimilars:
Not for the Faint of Heart
Discover the 4 key challenges you’ll need to overcome for biosimilar success, and how Avid is further expanding its capacity to meet growing customer demand.

Please enter your contact information to download the article. Fields marked with a * are required.